Aluminum (Al) and its alloys are widely used in numerous technological applications, each requiring a different combination of physical and chemical properties. Among various properties of pure Al, the corrosion resistance is important one. Although Al is placed in galvanic series with reactive metals, Al exhibits low corrosion due to formation of a thin, inert, and self-healing surface oxide layer. If the oxide layer on Al surface is damaged, a new passive layer forms immediately to provide corrosion resistance. Generally, Al and its alloys have good corrosion resistance in near neutral pH conditions. However, in acidic and alkaline conditions aluminum oxide is soluble and it ruptures in chloride, fluoride, and iodide environments triggering localized corrosion attack known as pitting corrosion that defeats the self-healing mechanism. Therefore, Al alloys need additional protection from such localized corrosion.
Pure Al (99.99 %) is very soft and has low strength. Its poor mechanical properties are the primary limiting factor for various structural applications. Alloying elements like Cu, Zn, Si, Mg, Fe, Cr, Li, Mn, and Sn enhance the mechanical properties of Al, but they often degrade the corrosion resistance significantly. Two points merit consideration in understanding corrosion of traditional Al alloys. Firstly, choice of alloying elements is based primarily on strength considerations. This ignores the formation of intermetallic precipitates (e.g. AlCuMg, AlCuFe) that interrupts the formation of oxide layer and enhances the corrosion rate. Secondly, solutes such as Mo, Ta, W, Nb, and Cr that are added to improve the corrosion resistance of Fe have very low solid solubility in Al (< 1 at %). Perhaps they also can be used to increase the corrosion resistance of Al and its alloys provided they are retained in the solid solution. Furthermore, formation of solid solution is difficult due to the differences in face centered cubic (FCC) crystal structure of Al and body centered cubic (BCC) structure of above alloying elements. Also, since the corrosion resistance of Al arises from the protective nature of the oxide film, the presence of solutes in the oxide film decreases aluminum’s susceptibility to pitting corrosion.
Rapid solidification and other non-equilibrium alloying methods such as sputter deposition, ion implantation, melt spinning, and vapor deposition can increase the solute solubility in alloys and enhance corrosion resistance. However, most of these methods need in-process high vacuum conditions and thereby impose restrictions on fabrication of complex structures, and thick coating (> 50μm), and many other limitations. On the contrary, surface modification via laser surface engineering (LSE) is a promising engineering approach to mitigate wear and corrosion problems. LSE has numerous advantages over the previously mentioned techniques. The precisely controlled heat input, rapid heating, and self-quenching can induce localized and homogeneous phase transformations in the microstructure. Using LSE, the intrinsic and extrinsic mechanical properties within selected regions of metallic components can be tailored without altering the bulk material properties.
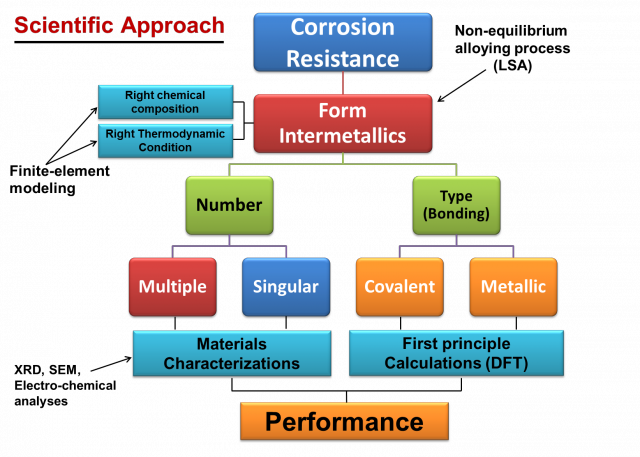
Figure 1 evaluates the fundamental scientific approach for the performance of laser alloyed surface during its application. Laser surface alloying (LSA) forms intermetallics, the formation of intermetallics mainly depend on the right chemical composition and thermodynamic condition (this can be studied using finite-element modeling). Further the intermetallics evolved after LSA can be classified into two parts. (i) Number of intermetallics (LSA can form multiple or singular), this can be evaluated from the XRD, SEM and electrochemical analysis. (ii) Type of intermetallic (can be covalent or metallic) can be evaluated using first principle calculations. In conclusion, these factors play an important role during the performance of intermetallics.
Preliminary results
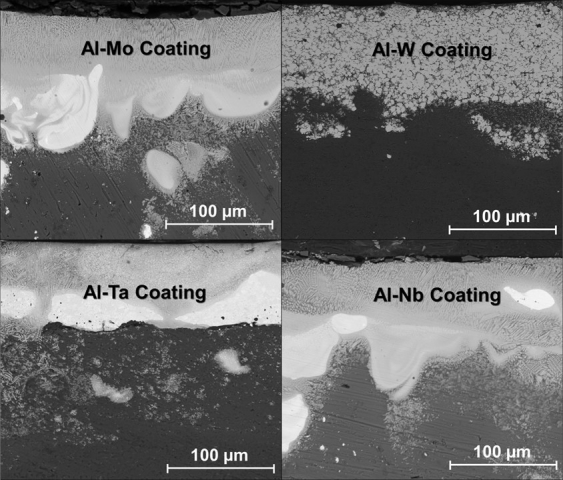
Microstructural Analyses: Back scattered cross sectional SEM micrographs of Mo, W, Ta and Nb coatings on Al 1100 samples obtained after laser processing (Figure 2) clearly indicate that the interface between the coating and substrate is continuous, adherent, and diffused with good metallurgical bonding.
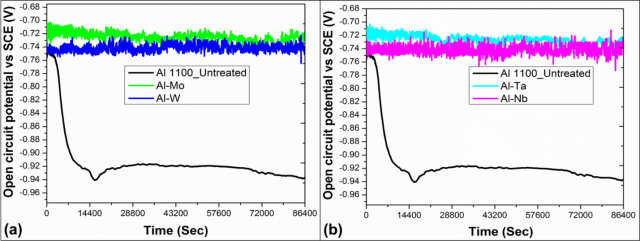
Corrosion Analyses: The measurement of open circuit potential (Figure 3) as a function of time indicates the most negative OCP over a 24 hour period. Sudden decrease and multiple fluctuations in the OCP curve likely points to thermodynamically instability of the oxide/passive layer on surface of Al 1100 is over the period of 24 hrs. On the other hand, the laser processed specimens showed a steady and stable value of OCP throughout the exposure time.
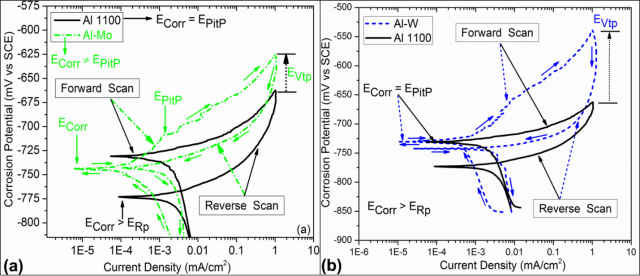
From the comparison of cyclic polarization behavior (Figure 4) of untreated and laser processed samples it is confirmed that Al 1100 exhibits the poor corrosion resistance mainly due to the high corrosion current density (i-Corr). The laser processed samples, on the other hand, show a substantial improvement in their corrosion resistance. This is attributed to the presence of thick alloyed region on the surface whose cathodic and anodic loop is displaced towards lower corrosion current densities.
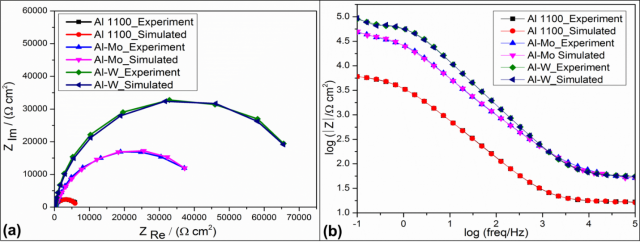
From the Nyquist plot (Fig 5a), it is clear that, for untreated and laser alloyed specimens capacitive loops are observed mainly due to the charge transfer resistance and double layer capacitance. However, laser alloyed specimens demonstrate a higher charge transfer resistance when compared with the untreated specimen (Al 1100).On the other hand, the Bode plot (Fig 5b) also shows the substantial increase in corrosion resistance after LSA mainly attributed to the increase in absolute impedance value |Z|.
Finite-Element Modeling: The in-situ measurement of dilution during the laser surface alloying process is an enormously difficult task, due to the localized nature of laser energy and very short laser-material interaction time. Therefore the computational approach (finite-element method and analysis of variance) was effectively employed to evaluate the dilution during the laser surface alloying process. A multiphysics (heat transfer + fluid flow) based computational model was developed to predict the dilution of molybdenum on an aluminum substrate during the laser surface alloying process. The influence of laser surface alloying processing parameters such as input energy, scanning speed, and overlapping ratio on dilution of Mo in Al was explored via computational model. The computational model, closely predicts the melt pool geometry (width and depth) that subsequently helps in estimating dilution. It was observed during laser surface alloying of Al with Mo that the dilution increases with the increase in laser power, while it decreases with the increase in scanning speed. The phase and microstructural analyses revealed the existence of Al5Mo intermetallic phases for most of the laser surface alloying processing conditions. These experimental observations validate the model’s predictions and points to its reliability in predicting the expected intermetallic phases in Al-Mo system for various laser surfacing alloying processing conditions.
Firstly, a finite-element model based on COMSOL™ multiphysics was developed to predict the dilution of Mo with Al during non-equilibrium laser surface alloying process. Secondly, the optimization model based on Design-Expert® was developed to find the optimal laser surface alloying parameters (laser power, scanning speed, and fill spacing) to obtain a microstructure suitable for improved corrosion resistance that is primarily attributed to the formation of Al5Mo intermetallic phase (16.7 at. % Mo). The main objectives of this work are to optimize laser surface alloying (LSA) parameters based on some targeted outcome such as dilution, microstructure, and corrosion properties; run the simulation (FEM) to find the optimal processing conditions to reach the desired (targeted) value; perform ANOVA for more accurate optimization; and lastly verify and validate experimental results via confirmation runs as illustrated in Figure 6.
The present optimization model utilized the prior experimental and computational (finite-element) modeling data for the concentration of Mo (at. %). The optimization analyses were carried out for the all the current datasets and the analysis revealed 44 optimal solutions that indicate the highest desirability. The confirmation runs were carried out to validate the optimization model. The experimental observation showed that the sample processed with optimal processing conditions demonstrates good corrosion resistance.
Optimization: From the previous investigation it was found that the formation of single homogeneous Al5Mo intermetallic phases (16.7 at. % Mo) within the laser surface alloyed characterized by XRD analysis clearly correlates with enhancement corrosion resistance. On the other hand, none of the LSA processing conditions used in the present computational model showed the exact 16.7 at. % Mo within LAR. Therefore, the current statistical model fixed 16.7 at. % Mo (corresponding to Al5Mo) as the optimal level of Mo concentration. In this case, the model used the optimization node of Design-Expert® V8 Software DOE to precisely predict the optimal LSA processing conditions. However, the present statistical model is capable of predicting any arbitrary concentration of Mo (at. %) and can be utilized to evaluate several other intermetallic phases of Al-Mo material system. The critical step of optimization is setting the optimization criteria. To find the 16.7% concentration of Mo within the LAR, the optimization “Target” criteria was set to be equal to 16.7 at. % Mo. The general factorial model predicted that both the laser power (56.83%) and scanning speed (30.17%) are two significant control factors (that predominantly influenced the response variable (concentration of Mo at. %). Thus, the optimization criteria for power and scanning speed was set as “In range” which are 400 to 1200 W for power and 10 to 100 mm/s for scanning speed.
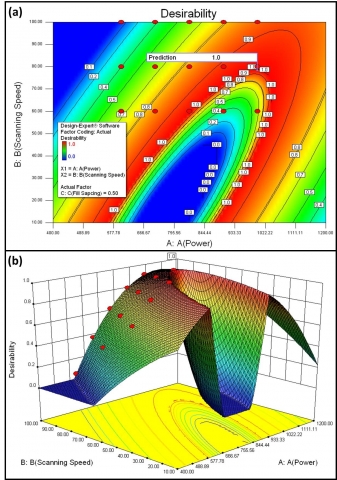
Myers and Montgomery described a multiple response mathematical method called desirability. This method combines multiple responses into one response called the desirability function, D(X), by choosing values from 0 (least desirable) to 1 (most desirable, all processing parameters are on target). The numerical optimization determined a point that maximized the desirability function. For one response (Mo at. %) and two factors (power and scanning speed), all goals are combined into one desirability function. The desirability of various LSA processing conditions (power = 400 to 1200 W, scanning speed = 10 to 100 mm/s, and fill spacing = 0.5 mm) was represented in two-dimensional and three-dimensional contour plot as illustrated in Figure 7. The desirability contour plot clearly distinguished the one distinct ‘sweet spots’ with a desirability value of 1.0 (Figure 7a). However, the three-dimensional contour plot (Figure 7b) of desirability gives more visibility to realize that the highest desirability was located in very narrow region (peaks of the mountain) and slight variation in processing conditions can lead to the adverse effect. In addition, the optimization analysis evidently showed the vicinity of desirability with the actual design points (filled circles) that clearly showed that none of LSA processing conditions has desirability of 1. Based on the selected optimization criteria (power = 400 to 1200 W, scanning speed = 10 to 100 mm/s, and fill spacing = 0.5 mm), the optimization analysis predicted total 44 sets of conditions.
Conclusions from the preliminary analysis
Laser surface alloying of transition metals on Al produced dense coatings with good metallurgical bonding was produced. The potential time measurements identify the more stable (steady state) potential values over long periods after laser surface alloying. Cyclic polarization and electrochemical impedance spectroscopy showed the substantial enhancement in the corrosion resistance after LSA when compared with untreated Al 1100. From the present computational (finite-element) model, the assessments of atomic percentage (at. %) of Mo, Ta, W, Nb and Al in the melt region was conducted that the prediction of possible intermetallic phases between Mo, Ta, W, Nb and Al for these combinations of atomic percentages. These computationally predicted phases were closely validated with the experimental observations (XRD, SEM, and EDS). The computational model predictions of melt pool width and depth, dilution, and intermetallic phases agreed well with experimental observations. Furthermore, Design-Expert® V8 Software for Design of Experiments (DOE) was also utilized to identify the optimal LSA processing parameters (laser power, scanning speed, and fill spacing) to obtain an improved corrosion and wear resistive surface. The results of these validation experiments indicated the close agreements between the predictions and observations.
Related Publications by the Group
- Ravi Shanker Rajamure, Hitesh D. Vora, S.G. Srinivasan, Narendra B. Dahotre “Laser Surface Alloying of Molybdenum on Aluminum for Enhanced Corrosion Resistance." Surface Coatings & Technology, Vol 258, pp. 337-342, 2014.
- Hitesh D. Vora, Ravi Sanker Rajmure, S. Santhanakrishnana, S.G. Srinivasan, Narendra B. Dahotre, "Design and optimization of microstructure for improved corrosion resistance in laser surface alloyed aluminum with molybdenum." International Journal of Precision Engineering and Manufacturing 2013, 14(8), 1421-1432.
- Hitesh D. Vora, Ravi Sanker Rajmure, S. Santhanakrishnana, S.G. Srinivasan, Narendra B. Dahotre, "Dilution of Molybdenum on Aluminum during Laser Surface Alloying." Journal of Alloys and Compounds 2013, 570, 133–143.
